In this study, we investigated the impact of concentration and temperature of precursors on the mechanical properties of sol-gel alumina doped with iron oxide. Samples were annealed at 1000 °C and at 1200 °C and exhibited molar ratios of Al2O3/Fe of 1/0.01, 1/0.05, 1/0.1, and 1/0.05. The latter sample was modified with glycerol at a molar ratio Al2O3/glycerol of 1/0.02. The molar ratio of Al2O3/H2O/EtOH/HNO3 used for the first three samples was 1/4/4/0.01, respectively, and for the last sample, the ratio was 1/4/4/0.02. Mechanical strength was characterized using uniaxial compression testing, and the microstructure was examined by scanning electron microscopy (SEM). The true density of the samples was measured using helium pycnometry, and the porosity was evaluated by mercury intrusion porosimetry (MIP). During the annealing process, between 1000 and 1200 °C, we observed a significant improvement in mechanical strength, which is attributed to the enhanced densification and crystallization of the alumina matrix.
Copper is one of the most extensively studied transition metal ions when embedded in an insulating matrix. The luminescent properties of monovalent copper and optical properties of copper nanoparticles have been widely investigated in various host matrices, including sol-gel silica glasses [1,2]. Copper can be incorporated into sol-gel silica glasses in three normal redox states: Cu2+, Cu+, and Cu0. Under oxidizing conditions, copper predominantly exists as cupric ions (Cu2+), imparting a blue-green color to the glass [3,4].
The redox equilibrium between Cu+ and Cu2+ shifts towards the cupric state with decreasing temperatures and reduced alkali content and basicity of the glass. During silica gel formation in air, Cu+ is oxidized to Cu2+ [1,3,5].
The copper ion species are uniformly incorporated into the sol-gel silica glass network, and the final optical properties are controlled by the initial purity and chemical homogeneity.
The sol-gel process involves simultaneous hydrolysis and polycondensation of an alkoxide, such as Si(OR)4, where R is an organic group. The structure of the condensed products depends on parameters like pH, alkoxide concentration, solvents, additives, dopants, temperature, pressure, and drying duration [6,7]. Sol-gel coatings prepared using copper and other dopants may offer a competitive alternative to traditional glass coloring methods. This study aims to evaluate the optical characteristics of sol-gel silica containing copper.
Preparation of Polymeric Silica Gels
Polymeric silica gels were synthesized through hydrolysis and condensation of Tetraethyl Orthosilicate (TEOS) with ethanol and deionized water, using nitric acid as a catalyst. Four samples (A, B, C, and D) were prepared with varying molar ratios of TEOS to copper nitrate (Cu(NO3)2·3H2O) as a dopant.
Sample Preparation
The molar ratios of TEOS to copper nitrate were 1/0.0084, 1/0.042, 1/0.084, and 1/0.0104 for samples A, B, C, and D, respectively. The final molar ratio of TEOS/H2O/EtOH/HNO3 was 1/3.8/3.8/0.005 for samples A-C and 1/3.8/3.8/0.009 for sample D. The initial pH of the sols was 1.5.
Synthesis Procedure
TEOS was added to a solution of water, ethanol, and nitric acid under vigorous stirring for 40 minutes. The mixture was then poured into plastic vials containing copper nitrate and stirred for 5 minutes to obtain a homogeneous solution.
Heat Treatment
Samples A-C were heated at 37, 51, 60, 70, and 80°C, while sample D, with added formamide, was heat-treated at 120°C.
Characterization
The samples were characterized by measuring:
True density using helium pycnometry
Porosity, specific surface area, and average pore diameter through nitrogen adsorption-desorption experiments
Optical transmission in the UV/vis/NIR spectral region using an integration sphere
IR transmission using a Bomem 102 spectrometer with KBr pellets in transmission mode
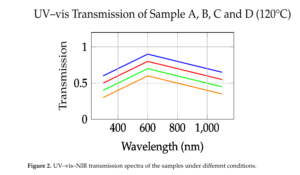
The UV/vis/NIR transmission spectra of the samples showed significant variations with increasing copper concentration and temperature. Samples A and B exhibited a strong cutoff in the UV region at 310 and 340 nm, respectively, while sample D displayed a transparency window from 300 nm after drying at 80°C, which shifted to 400 nm after heating at 120°C. Furthermore, the transmission band narrowed, and the absorption band broadened with increasing copper concentration, indicating a significant impact of copper doping on the optical properties of the silica gels [8].
The structural properties of the samples, including specific surface area, specific pore volume, and average pore diameter, were also investigated. All samples exhibited fine pore structures, with sample D showing a smaller specific surface area, suggesting a different path of pore structure evolution. This difference is attributed to the presence of formamide in sample D, which affected the kinetic models of aggregation and led to more linear structures [9].
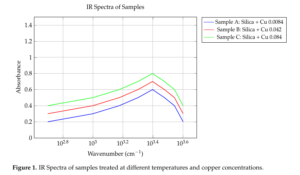
The FT-IR study revealed valuable information about the structural modifications in the silica network. The asymmetrical stretching (S) band at around 1078 cm\(^{-1}\) and stretching band at around 970 cm\(^{-1}\) indicated the presence of transversal optical (TO) bands associated with “rocking” (R), “bending” (B), and “asymmetrical stretch” (S) modes [10]. Additionally, the longitudinal optical (LO) resonance at 1250 cm\(^{-1}\) suggested structural disorder and modifications in the silica network. Notably, the decrease in the nitrate peak after drying at 120°C indicated changes in Cu2+ ion coordination, leading to the observed color change.
Overall, the results demonstrate the significant impact of copper doping and temperature on the structural and optical properties of silica gels. The findings provide insights into the mechanisms of gel formation, pore structure evolution, and the role of copper ions in modifying the silica network, highlighting the potential applications of these materials in various fields [11].
The broadening of the absorption band and shifting of the maximum peak of transmission with increasing copper concentration can be attributed to the increased scattering of light by the copper ions. This is consistent with the observed color change from blue to green with increasing copper concentration. The temperature-dependent transmission spectra also suggest that the copper ions play a crucial role in modifying the optical properties of the silica gels.
The structural properties of the samples, including specific surface area, specific pore volume, and average pore diameter, are consistent with the kinetic models of aggregation. The presence of formamide in sample D affects the pore structure evolution, leading to a smaller specific surface area and a more linear structure. This suggests that the addition of formamide can modify the gelation process and lead to materials with different properties [11].
The FT-IR study provides valuable information about the structural modifications in the silica network. The shift in the (S) band to higher wavenumbers indicates an incomplete hybridization and formation of linear or randomly branched polymers. The presence of TO bands and LO resonance suggests structural disorder and modifications in the silica network. The decrease in the nitrate peak after drying at 120°C indicates changes in Cu2+ ion coordination, leading to the observed color change. This suggests that the copper ions are coordinated with the silica network and play a crucial role in modifying its structure [12,13].
Overall, the results demonstrate the significant impact of copper doping and temperature on the structural and optical properties of silica gels. The findings provide insights into the mechanisms of gel formation, pore structure evolution, and the role of copper ions in modifying the silica network. This knowledge can be used to design and synthesize materials with specific properties for various applications.
This study has investigated the effects of copper doping and temperature on the structural and optical properties of silica gels. The results show that copper doping significantly affects the optical properties of the silica gels, leading to changes in transmission spectra and color. The structural properties of the samples, including specific surface area, specific pore volume, and average pore diameter, are also affected by copper doping and temperature. The FT-IR study reveals structural modifications in the silica network, including changes in Cu2+ ion coordination, leading to the observed color change. Overall, this study demonstrates the potential of copper-doped silica gels for various applications, including optical materials, sensors, and catalysts. Further research can focus on optimizing the copper doping concentration and temperature to achieve specific properties for targeted applications.