An experimental study on the friction and wear properties of ceramic materials (\(Al_2O_3\), \(ZrO_2\), \(Si_3N_4\), SiC) in contact with an \(Al_2O_3\) ball was conducted in vacuum and HFC-134a gas environments. XPS analysis of wear tracks and debris revealed reduced friction and wear in HFC-134a gas, with ionic ceramics exhibiting lower friction and wear. The formation of metal fluorides and fluorine-containing compounds on sliding surfaces was attributed to tribochemical reactions, dependent on ceramic bond type.
Advanced engineering ceramics possess high hardness, high resistance to high temperature and corrosion, making them suitable for tribological applications, especially in severe environments [1]. However, the lack of understanding of ceramic lubrication mechanisms limits their industrial use. The friction and wear behavior of ceramics are strongly dependent on the surrounding atmosphere, which can be attributed to the products of tribochemical reactions and the formation of reactive transfer films.
Studies have explored the lubrication effects of various liquids and gases on ceramic materials, revealing that the surrounding materials can be used as a source of lubricants. For instance, oxygen has been found to lubricate SiC by forming silica and releasing graphite-like material, while benzene and acetone vapors form sticky reaction products that reduce the friction and wear of \(ZrO_2\). Despite these findings, the explanations for reduced friction and wear vary for different ceramics[2].
This research aims to investigate the friction and wear behavior of different ceramic disks sliding against an \(Al_2O_3\) ball in a vacuum and in CF3CH2F (HFC-134a) gas. The surface composition and chemical state of the wear tracks and debris on the disks will be analyzed using X-ray photoelectron spectroscopy (XPS). The objectives are to clarify the possibility of fluorine-containing gas lubricating different ceramic couples and to understand the role of tribochemical reaction products in their tribological behavior. This knowledge is crucial for expanding the use of ceramics as tribomaterials in various fields[3].
Their dimensions measured 25.0 ± 0.1mm in diameter and 7.5 ± 0.7mm in thickness. The ball, with a diameter of 6.35 mm, was a commercially available product. Before conducting the friction tests, the specimens were cleaned in an ultrasonic bath using acetone for 10 minutes, followed by hexane for 10 minutes. The elemental composition of the specimen surfaces is detailed in Table 1. In addition to the elements inherent to the materials, a significant amount of C and O, likely originating from organic contaminants, was detected on all surfaces despite the cleaning process [4]. Furthermore, except for the \(ZrO_2\) disk, Al, Si, and N were also detected on the other specimens, possibly due to the presence of \(Al_2O_3\) and/or \(Si_3N_4\), which are commonly used as ceramic binders. The gas lubricant chosen was CF3CH2F (HFC-134a, purity >99%), a small fluorine-containing molecule currently utilized as a hydrofluorocarbon coolant in refrigeration systems.
| Specimen | Ball | Disk 1 | Disk 2 | Disk 3 | Disk 4 |
|---|---|---|---|---|---|
| Material | Al\(_2\)O\(_3\) | Al\(_2\)O\(_3\) | ZrO\(_2\) | Si\(_3\)N\(_4\) | SiC |
| Density (g/cm3) | 3.85 | 3.95 | 6.20 | 3.25 | 3.30 |
| Vickers Hardness (kg/mm2) | 1680 | 1670 | 1560 | 1320 | 3200 |
| Surface Roughness, Ra (m) | 0.020 | 0.08 | 0.09 | 0.05 | 0.35 |
The device comprises two high vacuum chambers. A ball-on-disk tribometer, designed to measure load and frictional force, was installed in the first chamber (refer to Figure 1). After the vacuum chamber was evacuated to a pressure of less than 10-5 Pa, gas at a fixed pressure was introduced via a variable leak valve, allowing the friction tests to be conducted in a well-controlled environment. Upon completion of the friction test, the first vacuum chamber was again evacuated to below 10-5 Pa, and the disk was directly transferred to the second ultrahigh vacuum system XPS analysis chamber (refer to Fig. 1) for surface analysis. The tribometer is a rotating ball-on-disk friction machine. The disk rotated at a speed of 240 rpm, corresponding to a sliding speed of approximately 0.30 m/s. Applied load and frictional force were monitored throughout the test using two pairs of strain-gage transducers. The applied load was 2.5 N, and each test was conducted over a sliding distance of 800 meters. In our previous study [18], HFC-134a gas at a pressure of 104 Pa demonstrated the most effective lubrication for an \(Al_2O_3\)/\(ZrO_2\) friction pair [5]. Therefore, a gas pressure of 104 Pa was used in this study as well. For comparison, friction tests were also performed in a vacuum of 10-5 Pa. All tests were conducted at room temperature. The surface profiles of the wear tracks on the disks were examined using profilometry. The wear volume of the disk specimens was estimated by measuring the diameter of the wear track and calculating the average cross-sectional groove area of the wear track from four measurements taken perpendicular to the sliding direction. The wear volume of the ball specimen was determined from the spherical cap calculated from an optical micrograph. A specific wear rate was defined as the total volume loss per unit load per unit sliding distance [6].
Elemental composition and chemical state analysis were conducted using micro-spot XPS. A monochromatic Al K\(\beta\) (1486.6 eV) X-ray source, along with a neutralization gun, was employed. To correct for the energy shift caused by charging effects, the C 1s peak from adventitious carbon was assumed to have a binding energy of 285.0 eV. A spot size of 120 \(\mu\)m in diameter was selected for XPS measurements, as it was smaller than the width of all the wear tracks [7].
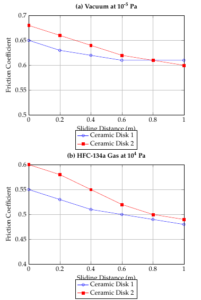
In Figure 2, the friction coefficients for various ceramic pairs sliding in a vacuum and in HFC-134a gas are displayed as a function of sliding distance. In the vacuum conditions shown in Figure 2, the initial friction coefficients range between 0.50 and 0.68. Regardless of the friction pairs, there is an initial transition phase during testing where the friction coefficient peaks and then stabilizes to a more consistent value. After 800 meters of sliding, the friction coefficients converge to approximately 0.61 ± 0.04, indicating no significant difference in friction when different ceramic disks slide against an \(Al_2O_3\) ball in a vacuum. Figure 2 demonstrates that HFC-134a gas significantly reduces friction across all ceramic pairs while also minimizing fluctuations. Ionic ceramic pairs exhibit lower friction in the presence of HFC-134a.
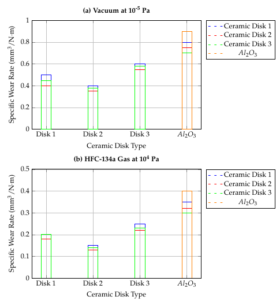
Figures 3 present the specific wear rates for the \(Al_2O_3\) ball and various ceramic disks in both vacuum and HFC-134a gas environments. It is observed that ionic ceramic pairs exhibit lower wear in both conditions, with HFC-134a gas reducing wear by approximately 1 to 4 orders of magnitude. The highest wear occurs when the \(Al_2O_3\) ball slides against the SiC disk in a vacuum, likely due to the SiC disk’s higher surface roughness (refer to Table 1), which causes greater abrasion of the ball.
Additionally, the wear tracks in vacuum and HFC-134a gas show distinct differences. Wear tracks formed in a vacuum are severely damaged, with a significant amount of wear debris surrounding them. XPS measurements reveal that the wear tracks are extensively covered with Al transferred from the \(Al_2O_3\) ball. In contrast, wear tracks formed in HFC-134a gas are smoother with minimal debris, particularly for the ionic ceramic pairs. These findings suggest that HFC-134a gas effectively lubricates ceramics, especially ionic ceramics.
Table 2 outlines the atomic concentrations of elements within the wear tracks on disks sliding in HFC-134a gas. The presence of the F element on all wear tracks indicates the formation of fluorides, likely due to tribochemical reactions between HFC-134a molecules and the ceramics. The chemical state changes due to these reactions were also investigated. The F 1s spectra within the wear tracks on disks sliding in HFC-134a gas are depicted in Figure 4. The binding energy of F 1s on the \(Al_2O_3\) disk is 687.0 eV, attributed to an overlap of AlF3 (686.3 eV) and C–F species (688.4–689.0 eV). The main F 1s peak on the \(ZrO_2\) disk is observed at a binding energy of 685.3 eV, corresponding to F 1s in ZrF4. The F 1s peaks on the \(Si_3N_4\) and SiC disks are similar and higher than those in Si–F (686.0–686.6 eV), indicating an overlap of F 1s in –SiFx compounds and in C–F species (688.4–689.0 eV). The shape of the F 1s peaks suggests that more organic fluorine-containing compounds form on the \(Si_3N_4\) disk.
| Disk Specimen | C | O | Si | N | Zr | Al | F |
|---|---|---|---|---|---|---|---|
| Al\(_2\)O\(_3\) | 42.3 | 18.6 | – | – | – | 9.5 | 5.3 |
| ZrO\(_2\) | 25.0 | 48.6 | – | – | 4.0 | 8.6 | 11.8 |
| Si\(_3\)N\(_4\) | 24.3 | 23.6 | 9.5 | 16.4 | – | 1.5 | 14.3 |
| SiC | 56.5 | 17.6 | 15.0 | – | – | 2.0 | 8.9 |
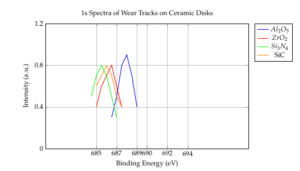
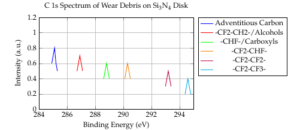
Figure 5 displays the C 1s spectrum of wear debris on the \(Si_3N_4\) disk. The C 1s spectrum can be divided into six peaks: adventitious carbon (284.9 eV, peak 1), –CF2–CH2–/alcohols (286.7 eV, peak 2), –CHF–/carboxyls (288.6 eV, peak 3), –CF2–CHF– (290.1 eV, peak 4), –CF2–CF2– (293.0 eV, peak 5), and –CF2–CF3– (294.4 eV, peak 6). This indicates that fluorine-containing polymers are generated on the frictional surface of the \(Si_3N_4\) disk when it slides against an \(Al_2O_3\) ball in HFC-134a gas.
Si 2p spectra, obtained from the wear tracks of silicon-based ceramics rubbing in HFC-134a gas. The main Si 2p peak on the \(Si_3N_4\) disk is observed at a binding energy of 101.8 eV, which corresponds to Si 2p in \(Si_3N_4\). A shoulder peak at 103.9 eV, attributed to Si–F, suggests the formation of –SiFx compounds. The Si 2p spectrum on the SiC disk consists of a main peak at 100.4 eV and a smaller peak at 103.9 eV, corresponding to Si 2p in SiC and –SiFx compounds, respectively.
Al 2p is detected on all disks sliding in HFC-134a gas, confirming the transfer of \(Al_2O_3\) from the ball during the sliding process. Based on the binding energy, it can be inferred that Al-oxyfluorides are present on the \(Al_2O_3\) and \(ZrO_2\) disks. Interestingly, \(Al_2O_3\) and AlF3 are found on the \(Si_3N_4\) and SiC disks, respectively, suggesting that the reaction of \(Al_2O_3\) to form AlF3 occurs more readily on the SiC surface than on \(Si_3N_4\). This may explain why the \(Al_2O_3\) ball shows the highest wear when sliding against the SiC disk in HFC-134a gas (Figure 3b).
The main products of tribochemical reactions between different ceramic disks and an \(Al_2O_3\) ball in HFC-134a gas are summarized in Table 4. Notably, organic compounds were not found on the typical ionic \(ZrO_2\) disk, whereas fluorine-containing polymers were clearly detected on the \(Si_3N_4\) disk. These results suggest that the products of tribochemical reactions are dependent on the bonding type of the ceramic disks.
Most surface reactions and the creation of surface intermediates involve the transfer of charge, either through electron transfer or proton transfer. These processes are often conceptualized as variations of acid-base reactions. \(Al_2O_3\) and \(ZrO_2\), recognized as solid acids, are known for their high catalytic activity. Research on the tribological behavior of fluorinated organic lubricants within an \(Al_2O_3\)–TiC head–disk interface has shown that these lubricants tend to decompose even under minimal load conditions. This decomposition is further accelerated by the formation of AlF3, a strong Lewis acid, as a tribochemical product. Similarly, the breakdown of perfluoropolyether (PFPE) lubricants on \(Al_2O_3\) surfaces, driven by Lewis acid sites, has been documented [3].
Using a specialized adsorption test apparatus, we observed the chemisorption of HFC-134a molecules at low pressure (10-4 Pa) on \(Al_2O_3\) and \(Si_3N_4\) surfaces, accompanied by the release of a tribochemical product, CHF=CF2. In this study, the presence of fluorine-containing polymers was notably detected on the \(Si_3N_4\) disk, suggesting that olefins are more prone to polymerization on the \(Si_3N_4\) surface compared to other surfaces [8].
Based on these observations, it can be inferred that friction generates Lewis acid sites on ionic ceramics. HFC-134a molecules undergo chemical adsorption and subsequent decomposition at these active sites, leading to the formation of metal-oxyfluorides. The development of Al–F and Zr–F species increases the reactivity of these sites, allowing the reactions to persist, ultimately producing AlF3 and ZrF4 [9]. In contrast, on covalent ceramics such as \(Si_3N_4\) and SiC, friction may generate radical sites. In addition to the formation of –SiFx compounds, fluorine-containing organic compounds are also produced, as olefin molecules are more readily polymerized at these radical sites. While polymerization can occur at Lewis acid sites, the process is more likely and efficient at radical sites [10].
Moreover, the reduced friction and wear observed in ionic ceramic pairs within HFC-134a gas could be attributed to the formation of metal fluorides, which potentially provide enhanced lubrication for the ceramic materials.
Based on the findings discussed, it can be concluded that the tribological behavior of ceramic materials in the presence of HFC-134a gas is significantly influenced by the surface chemistry and the nature of the ceramics. The study demonstrates that ionic ceramics, such as \(Al_2O_3\) and \(ZrO_2\), exhibit enhanced lubrication and reduced wear due to the formation of metal fluorides like AlF3 and ZrF4. These metal fluorides, formed at Lewis acid sites generated by friction, contribute to a lower friction coefficient and improved wear resistance.
In contrast, covalent ceramics like \(Si_3N_4\) and SiC tend to form both –SiFx compounds and fluorine-containing organic polymers. These materials, influenced by radical sites generated during friction, also contribute to the overall tribochemical processes, but their mechanisms differ from those of ionic ceramics.
The distinct surface reactions, driven by the intrinsic chemical properties of the ceramics, underline the importance of understanding the material-specific interactions with lubricants like HFC-134a. The formation of tribochemical products, especially in ionic ceramics, not only enhances lubrication but also suggests potential avenues for optimizing material performance in environments where low friction and wear are critical.
Overall, the study underscores the significance of tailoring lubricants and surface treatments to specific ceramic materials, considering their unique chemical behaviors and the resulting tribochemical products, to achieve optimal tribological performance.