Adhesion between microparts and grippers significantly hinders precise manipulation in both ambient and vacuum environments. This study aims to develop an optimal coating for silicon microgrippers to mitigate these sticking effects. By employing an atomic force microscope (AFM) tip as a model gripper and varying surface conditions, we investigated the underlying mechanisms of adhesion, primarily attributed to capillary forces on oxide surfaces. While hydrophobic coatings effectively reduce capillary forces, they can inadvertently increase adhesion due to enhanced contact area and potential contamination. Our AFM-based methodology enables the evaluation of coating suitability. Preliminary results suggest that surface structuring holds promise for further diminishing van der Waals forces and consequently, overall adhesion.
Precise manipulation of objects at the micrometer scale becomes difficult due to adhesion forces exceeding gravity. This prevents accurate placement when the object adheres more strongly to the gripper than the target surface. To address this limitation, this study explores antiadhesive coatings for microgrippers. We utilize an Atomic Force Microscope (AFM) tip as a model gripper and investigate interactions with various surfaces representing the workpiece [1,2]. AFM, initially designed for high-resolution imaging of insulating surfaces, has become a valuable tool for characterizing material properties like friction, elasticity, and surface forces.
Force-distance curves (FDCs) obtained by recording cantilever deflection provide insights into adhesion forces at small separation distances. While liquid bridges can increase adhesion, specific solutions can also reduce it. Both attractive and repulsive interaction forces within FDCs have been extensively studied theoretically and experimentally. This work focuses on identifying a suitable coating for a microfabricated gripper operating in ambient conditions and observed under a stereo light microscope [3].
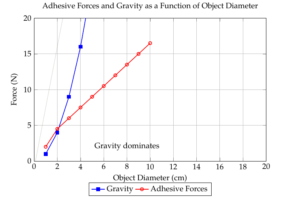
The burgeoning field of microrobotics holds immense promise for revolutionizing various industries, from medicine to manufacturing. However, the miniaturization of components brings forth unique challenges, with adhesion forces becoming a dominant factor in manipulating microscopic objects. Unlike larger-scale operations where gravity typically overcomes adhesive forces, these forces become paramount at the micro-scale, hindering precise and controlled manipulation. When a microgripper attempts to release a workpiece, the adhesive bond often proves stronger than the desired placement, resulting in failures and inefficiencies. To address this critical issue, the development of anti-adhesive coatings for microgrippers is imperative [4]. This study aims to investigate the efficacy of different coatings in minimizing adhesion forces and enhancing the performance of microgrippers.
By employing an atomic force microscope (AFM) as a model system, we can meticulously analyze the interactions between coated surfaces and explore the underlying mechanisms of adhesion. The insights gained from this research will contribute to the advancement of micromanipulation techniques and enable the development of more sophisticated micro-scale systems [5].
The convergence of materials science, engineering, and nanotechnology has ushered in an era of unprecedented miniaturization. At the forefront of this revolution lies the field of microrobotics, which promises to reshape industries from healthcare to manufacturing. Microrobots, with their diminutive size and unparalleled precision, have the potential to perform tasks that are unattainable through conventional methods.
However, the miniaturization of components brings forth unique challenges that must be overcome to fully realize the potential of microrobotics. One of the most significant hurdles is the dominance of adhesive forces over gravitational forces at the micro-scale. This phenomenon drastically impacts the manipulation of micro-objects, as traditional handling techniques become ineffective. When a microgripper attempts to release a workpiece, the adhesive bond often proves stronger than the desired placement, leading to errors and inefficiencies.
To address this critical challenge, the development of anti-adhesive coatings for microgrippers is essential. By minimizing the adhesive forces between the gripper and the workpiece, these coatings can significantly enhance the precision and reliability of micromanipulation processes. This study aims to investigate the efficacy of various coatings in mitigating adhesion and advancing the capabilities of microrobotics.
Our experimental procedure consisted of creating force-distance curves (FDCs) by gradually moving the tip towards the surface and subsequently retracting it. The maximum force required to separate the tip from the surface was identified as the adhesion force. Prior to the measurements, samples underwent a cleaning process using ultrapure water, followed by potential heating to eliminate any residual water molecules. It is important to note that hydrophobic coatings can introduce complications into the measurement process by contaminating the tip. This contamination can adversely affect the accuracy of the adhesion force measurements due to changes in the tip’s geometry.
The experimental setup relied on an ultra-high vacuum scanning tunneling microscope and atomic force microscope (UHV-STMrAFM) system. This instrument provided the necessary precision and controlled environment for the adhesion force measurements. Commercial silicon nitride tips, characterized by a spring constant of 0.12 N/m and a tip radius in the range of 20 to 50 nanometers, were employed for the experiments. To accurately quantify the force exerted between the tip and the sample surface, infrared beam deflection was utilized, offering a force resolution of 20 to 50 piconewtons (pN).
The experimental protocol involved acquiring force-distance curves (FDCs). This was achieved by gradually approaching the tip towards the sample surface and subsequently retracting it. By analyzing these curves, the maximum force required to overcome the adhesive interaction and separate the tip from the surface was determined, thereby quantifying the adhesion force. Prior to conducting the adhesion measurements, the samples underwent a rigorous cleaning process involving the use of ultrapure water. In certain cases, additional sample treatment, such as heating, was implemented to ensure the removal of any residual water molecules that could potentially interfere with the adhesion measurements.
It is crucial to acknowledge the potential challenges associated with hydrophobic coatings. These coatings have a propensity to contaminate the AFM tip, which can significantly impact the accuracy of the adhesion force measurements. The contamination alters the tip’s geometry, thereby influencing the interaction forces and compromising the reliability of the obtained data.
Even in vacuum environments, we observed substantial adhesive forces, reaching several hundred nanonewtons, for a range of oxidized materials including silicon, aluminum, silver, and platinum. To gain a deeper understanding of coating behavior, we conducted a series of adhesion measurements.
Initially, we determined the adhesion force (FSiO2cal) between a pristine tip and a dehydrated silicon oxide surface. Subsequently, the adhesion force (Fsample) of each uncontaminated sample was measured using the same clean tip. To investigate the impact of tip contamination, we remeasured the adhesion between the contaminated tip and silicon oxide (FSiO2cont), comparing the results to the initial measurement (FSiO2cal). Furthermore, we assessed the reversibility of contamination by measuring FSiO2cal again after scanning with the contaminated tip. The obtained data are summarized in Table 1.
| \(F^SiO_2_cal\) (nN) | \(F^SiO_2_uncount\)(nN) | \(F^SiO_2_count\)(nN) | \(F^SiO_2_scann\)(nN) | Comments |
| 55 | 16.4 | 170.0 | 74.4 |
Heating out required, contamination
not removable by scanning |
| 55 | 18.4 | 55.2 | 55.2 | No contamination |
| 55 | 33.2 | 84.2 | 55.2 |
Contamination removable
by scanning |
| 35 | 22.2 | 110.2 | 65.2 |
Not completely removable
contamination |
| 26 | 13.4 | 155.5 | 155.5 |
Huge contamination,
not removable |
| 23.10 | 300.0 | 19.1 | 19 |
Not completely removable
contamination leads to a reduced adhesion |
| 58 | 25.5 | 200.0 | 58.3 | No contamination |
Our analysis revealed that water desorption from the Altefco sample (PTFE embedded in aluminum oxide) was incomplete at room temperature. Prior to annealing, we measured adhesive forces of 170 nN, characteristic of liquid bridges. The FEP1 and FEP2 samples, composed of perfluoroethylene-propylene, were coated on aluminum oxide and subsequently sintered at 250°C and 200°C, respectively. Based on the data in Table 1, additional annealing was deemed unnecessary. While the stiffer FEP2 coating did not contaminate the tip, the contamination from FEP1 could be readily removed.
The force-distance curve (FDC) of a silicone-contaminated tip on a SiO2 surface exhibited a distinct feature: a sudden change in slope at a consistent separation distance. This behavior suggested the formation of a capillary by the silicone contamination prior to detachment from the SiO2 surface. Removing this silicone contamination from the tip proved challenging. The PTFE sample demonstrated similar behavior, with significant contamination that was partially removable through scanning. In contrast, a plasma-deposited 50 Å PTFE layer on native SiO2 resulted in irreversible tip contamination and a substantial increase in adhesion due to an enlarged tip radius. The amorphous carbon-coated sample effectively reduced adhesion by at least a factor of two while preventing tip contamination. However, physisorbed water required removal through mechanical or annealing processes.
To explore the effect of surface roughness, we deposited a 180 nm gold film on silicon oxide. The resulting surface exhibited a corrugated topography. The tip was expected to encounter adhesion forces varying between those of two planar surfaces (in valleys) and two spheres (on protrusions).
Spatially unresolved FDC measurements using three cantilevers with different tip radii revealed variations in adhesion forces (Fgoldadh) on the gold surface compared to the reference SiO2 surface (FSiO2cal). While FSiO2cal remained relatively constant, Fgoldadh exhibited fluctuations of up to a factor of 3.5 to 4, attributed to the surface roughness.
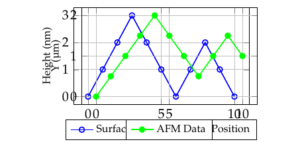
To confirm the influence of topography, we conducted spatially resolved measurements using the cantilever with the closest FSiO2cal value to 55 nN. Figure 2 presents the selected area, tip position, and surface line profile. Adhesion forces were measured at the top of a protrusion (Fgoldadh = 9 ± 1 nN) and in a valley (Fgoldadh = 19 ± 1 nN), corresponding to positions 80 nm and 40 nm, respectively, as indicated by the black bar in Figure 6. The observed difference in adhesion forces clearly demonstrates the impact of surface topography. Improving the protrusion shape is expected to further reduce adhesion.
The influence of water on adhesion is evident from the Altefco sample, where significant adhesive forces were attributed to liquid bridges [8]. This emphasizes the importance of thorough sample preparation, including effective water removal, to accurately assess the intrinsic adhesive properties of materials [9]. The successful removal of water from the FEP samples through sintering demonstrates the feasibility of this approach for certain coating systems.
The impact of tip contamination on adhesion measurements is a crucial consideration [10]. The observed increase in adhesion forces after tip contamination with silicone and PTFE highlights the need for careful experimental design and potential contamination control measures. While amorphous carbon coatings appear promising in terms of reducing adhesion and preventing tip contamination, the presence of physisorbed water necessitates additional treatment steps [11].
The results obtained from the surface roughened gold samples demonstrate the complex interplay between topography and adhesion. The observed variations in adhesion forces across the surface highlight the importance of considering surface morphology when designing microgrippers. The correlation between adhesion and topographical features suggests that optimizing surface roughness could be a viable strategy for reducing adhesion [12].
Our findings contribute to a deeper understanding of the factors influencing adhesion at the micro-scale. The identification of coating materials with reduced adhesive properties and the exploration of surface modification techniques represent significant steps towards overcoming the challenges associated with micromanipulation. However, further research is required to develop robust and scalable anti-adhesive solutions for practical applications [13-15].
This study underscores the critical role of adhesive forces in impeding efficient micromanipulation, even in controlled vacuum environments. By employing AFM-based measurements, we have elucidated the significant impact of water contamination on adhesion, emphasizing the need for rigorous sample preparation. While hydrophobic coatings offer potential benefits, their effectiveness is often compromised by increased contact area and tip contamination.
Our findings highlight the complex interplay between surface chemistry, topography, and adhesion forces. The observed correlation between surface roughness and adhesion suggests that surface engineering can be a promising approach to mitigating adhesion challenges. However, further research is necessary to develop comprehensive anti-adhesive strategies that address the multifaceted nature of this problem.
In conclusion, this study provides valuable insights into the mechanisms underlying adhesion at the micro-scale. By understanding the factors influencing adhesive interactions, we can develop more effective anti-adhesive solutions and advance the field of microrobotics. Future research should focus on exploring novel materials, surface modifications, and characterization techniques to overcome the limitations of current approaches.